Our Hip Products
Almond Modular Cementless Stem
Modular Cementless Stem is a well proven implant. It consists of a complete set for primary total hip replacement:
The femoral stem is designed to guarantee an optimal metaphyseal filling.
The metaphyseal zone has a triangular section to avoid the generation of varus forces.
The triangular section in the metaphiseal zone guarantees an antirotational effect and theround section in the bottom stem part allows an optimal medullary canal filling.
The internal stem profile changes with the implant size in order to keep constant the head offset and avoid a valgisant effect for the smaller sizes.
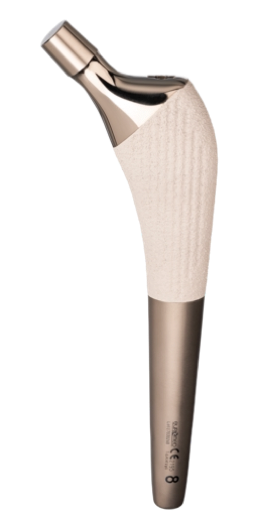
Material:
Certified TI6AI4V ELI according to ASTM F 136 – ISO 5832/3
Almond Modular Cemented Stem
The Almond Modular Cemented Stem is a well proven implant. It consists of a complete set for primary total hip replacement:
The femoral stem is designed to guarantee an optimal metaphyseal filling.
The metaphyseal zone has a triangular section to avoid the generation of varus forces.
The triangular section in the metaphiseal zone guarantees an antirotational effect and the round section in the bottom stem part allows an optimal medullary canal filling.
The internal stem profile changes with the implant size in order to keep constant the head offset and avoid a valgisant effect for the smaller sizes.
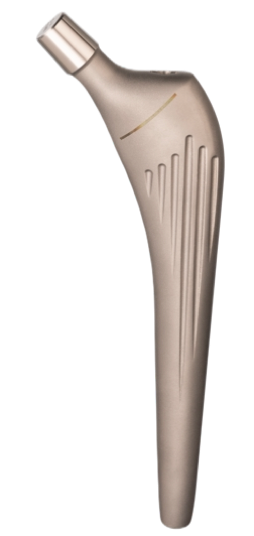
Material:
Certified CoCrMo alloy according to ASTM F 75 – ISO 5832/4
Modular Straight Cementless Stem
The modular straight cementless stem hip prosthesis is designed to be used as a primary and a revision total hip. The stem sizes allow accurate matching of prosthesis to the medullary canal. The prosthesis offers dual fixation by combining the locking effect of a stem to bone contact, plus minimal bone cement support. It may be used in conjunction with the modular head cone 12/14 and polyethylene acetabular components for total articular replacement.
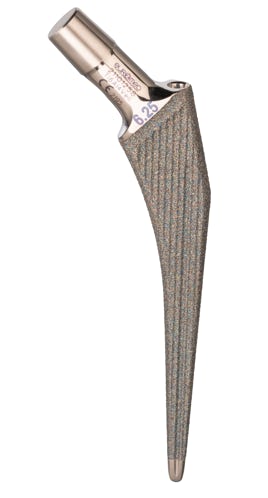
Material:
Certified TI6AI4V ELI according to ASTM F 136 ISO 5832/3
Modular Straight Cemented Stem
The modular straight cemented stem hip prosthesis is designed to be used as a primary and revision total hip. The 4 stem sizes allow accurate matching of prosthesis to the medullary canal. The prosthesis offers dual fixation by combinig the locking effect of a stem to bone contact, plus minimal bone cement support. May be used in conjuction with modular head cone 12/14 and polyethylene acetabular components for total articular replacement.

Material:
CoCrMo alloy according to ASTM F 75 – ISO 5832/4
EUROFIT Primary Cementless Stem
Eurofit Primary Cementless Stem is a well proven implant, which is designed to be used as a primary total hip. The stem sizes allow accurate matching of prosthesis to the medullary canal. The prosthesis offers fixation locking effect of a stem to bone contact. This particular stem has vertical and horizontal shapes with tapered distal edges to increase stability. It may be used in conjuction with the modular head cone 12/14 and Optifit Cementless acetabular cup or cemented cup for total articular hip replacement.

Material:
Certified TI6AI4V ELI according to ASTM F 136 ISO 5832/3
EUROFIT Revision Cementless Stem

Eurofit Revision Cementless Stem is a well proven implant, which is designed to be used as a revision total hip. The stem sizes allow accurate matching of prosthesis to the medullary canal. The prosthesis offers fixation locking effect of a stem to bone contact. This particular stem has vertical and horizontal shapes with tapered distal edges to increase stability. It may be used in conjuction with the modular head cone 12/14 and Optifit Cementless acetabular cup or cemented cup for total articular hip replacement.
Material:
Certified TI6AI4V ELI according to ASTM F 136 ISO 5832/3
Cemented Cup
The cups are placed in degenerated anatomical acetabulum and requires cement for fixation. Provided with radiopaque wire for postoperatory visualisation. The standard 50 mm acetabular cup meets the anatomical requirements of major patients, while the 44 mm cup is used if the ilium is narrow in cases of high dislocation with migration of the acetabulum. Two larger sizes of acetabular cups, 54, 58 mm have been added to the range to meet special requirements of large boned patients. The U.H.M.W. polyethylene cup has wellproved tissue tolerance, elasticity and a very low coefficient of friction.

Particular attention is paid to the precision machining and surface finish of the acetabular component, so that, when combined with femoral component, and exceptionally stable total hip replacement is achieved with minimal risk of dislocation and minimum wear rate on the acetabular component.
Material:
XL UHMWPE (Cross-Linked Ultra High Molecular Weight Polyethylene) according to ISO 5834/1-2 selected for its high degree of purity, good biotolerance, high mechanical performance and friction properties.
Bipolar Head
Placement of cup together with a femoral head on stem gives an articulated partial prosthesis. The modular outher shell is manufactured from certified stainless steel and inner side, the insert is manufactured from Ultra High Molecular Weight Polyetylene UHMWPE.
Material:
XL UHMWPE (Cross-Linked Ultra High Molecular Weight Polyethylene) according to ISO 5834/1-2 selected for its high
degree of purity, good biotolerance, high mechanical performance and friction properties.
Metal cup made from Certified Stainless Steel according to ASTM F 138 – ISO 5832/1 or CoCrMo alloy according to ASTM F75 – ISO 5832/4.

Optifit Cementless Cup
In principal for all forms of arthrosis, advance wear and tear of the hip joint because of degeneratif, posttraumatic or rheumatoid arthritis fracture of avascular necrosis of the head of femur. Sequelae of earlier operations such as osteosynthesis, joint reconstruction, arthrodesis, hemiarthroplastic or total hip replacement. Cementless Standard Cup is an acetabular implant for uncemented fixation. The standard cup made of Ti6Al4V alloy with three dimensional interlock grit blast presents a hemispherical shape with polar deflection to enhance the perifery distribution of the stresses effecting the cup. The titanium cup has three holes on supero-lateral side. The screws have a spheric head and have always a good contact with their seatings, even if the introduction angle may be different. This prevents the fratting corrosion and assures the screw stability. In spite of the different possible introduction angles of the screw. Choosen as the basic material was Ti6Al4V which has been tried and tested over many years and is outstandingly biocompatible. For the bone side contact surface optional Titanium Plasma Spray coating or additional Hydroxyapatite coating enhances initial fixation and long-term bone integration. The porosity of plasma spray is ideal for osseointegration.

Material:
Certified TI6AI4V ELI according to ASTM F 136 – ISO 5832/3.
Optifit PE Crosslinked Liner
The cementless standard cup can reduce the possibility of wear debris from the inner surface of the UHMWPE cup. It is done by machining and polishing of the inner surface. The good precision of manufacture assures more over the direct contact between the UHMWPE insert and inner surface of the metal cup reducing micromotions and fretting.
Material:
XL UHMWPE (Cross-Linked Ultra High Molecular Weight Polyethylene) according to ISO 5834/1-2 selected for its high
degree of purity, good biotolerance, high mechanical performance and friction properties.
Ceramic insert Bioceral® Alumina according to ISO 6474 by HTI Technologies (France)

Optifit Ceramic Liner
Acetabular implants for endoprosthetics made of high-performance ceramic material have proven themselves well in clinical use for decades and can be used with all common prosthesis materials can be combined.
Ceramic pan inserts enable one due to their good breaking strength extremely wear-resistant movement sequence in modern endoprosthesis systems.

Ball head
The placement of the head on the stem gives an articulated partial prosthesis. The head is set on the taper of the stem giving required movement within the inner semisphere of the acetabulum.
Acetabular implants for endoprosthetics made from modern high-performance materials
have proven themselves well in clinical use for decades and can be used with all common prosthesis materials combined.
These material has excellent bio mechanical properties and ensure maximum functionality and reability for the patient
Material:
• Certified CoCrMo alloy according to ASTM F 75ISO 5832/4.
• Certified Stainless Steel according to ASTM F 138 ISO 5832/1
• Ceramic head Bioceral® Alumina according to ISO6474 by HTI Technologies( France)
• Ceramic head ELEC® plus according to ISO6474


Our Products


The name Euromed stands for high-quality joint implants and surgical instruments. Learn more about our Products.
Contact us
Euromed Implants GmbH
Headquarter
Bei St. Wilhadi 1
21682 Stade
Germany
Warehouse
Wilhadi-Kirchhof 13
21682 Stade
Germany
Training Center
Beguinenstr. 27
21682 Stade
Germany
Fax +49 (0) 4141 7782 700
© 2024 - Euromed Implants GmbH
b. St. Wilhadi 1, 21682 Stade, Germany office@euromed-implants.de Tel. +49 (0) 4141 7897 290


